Describe a Phospholipid Molecule and Its Interaction With Water
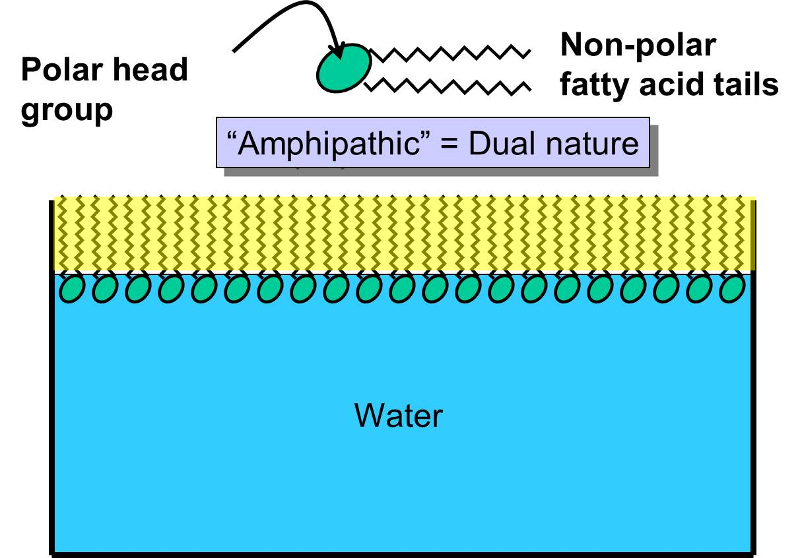
Describe a phospholipid molecule and its interaction with water. Consists of a polar end containing a phosphate group that is hydro-phillic and a non-polar end containing two fatty acids that is hydrophobic.
Overview Of Cell Structure And Function
Experts are tested by Chegg as specialists in their subject area.

. A phospholipid is made up of two fatty acid tails and a phosphate group head. The second group the hydrophobic tail consists of two fatty acid chains. Hydrophilic water-loving polar properties of phospholipids found in.
The hydrophilic regions of the phospholipids tend to form hydrogen bonds with water and other polar molecules on. Phospholipids to form a bilayer where the head regions face the surrounding water molecules and the opposing tails face each other. Phospholipids have two major components - a polar head hydrophilic and a non-polar tail hydrophobic.
View the full answer. Because phospholipids have a polar head the phosphate and a nonpolar tail the lipids they are amphipathic. When it is placed in water the phospholipid molecules form a.
The head is hydrophilic and the two tails are hydrophobic. Some species use three fatty acid chains but two is most common. What is a phospholipid molecule interaction with water.
Phospholipids or Phosphatides are amphiphilic a chemical compound containing both lipophilic or water-loving and hydrophilic or water-fearing properties lipids. B- a fat molecule is less soluble in water because it has more carbons and hydrogens than a phospholipid. Which of the following statements describes the interaction of water molecules with phospholipids.
Composed of glycerol linked to two fatty acids and a phosphate. Fatty acids are long chains that are mostly made up of hydrogen and carbon while phosphate groups consist of a phosphorus molecule with four oxygen molecules attached. In contrast the phospholipid tails are hydrophobic repelled by water molecules.
These groups are polar and are attracted to water. The phospholipid heads are hydrophilic attracted to water molecules. We review their content and use your feedback to keep the quality high.
The polar heads interact with water the non-polar tails do not. Describe a phospholipid -molecule and its interaction with water consists of a polar end containing a phosphate group. Phospholipids are composed of a glycerol and phosphate group hydrophilic head and two fatty acids hydrophobic tails.
A phospholipid contains an ionic head that contains the phosphate group and the nonpolar or hydrophilic tail that is comprised of the alkyl moeity in the phospholipid. Thus if you drop phospholipids in water often they will group up in beads called micelles. C- a phospholipid is less soluble in water because it is smaller than a fat molecule.
These two components of the phospholipid are connected via a third molecule glycerol. Since water is a polar molecule the hydrophilic. The hydrophilic head consists of a glycerol molecule bound to a phosphate group.
The nonpolar tails attract water because water is polar and opposites attract. The hydrophilic ionic head can interact with water while the hydrophilic alkyl tail do not interact with water hence the answer above. Describe a phospholipid molecule and its interaction with water.
In our laboratory we have studied the water adsorption of various phospholipids with a view towards providing information relevant to the biological function of the hydrated lipids. How do phospholipids interact with water molecule. A phospholipid consists of two basic parts.
A phospholipid is an amphipathic molecule which means it has both a hydrophobic and a hydrophilic component. The phospholipid head is hydrophilic hence is attracted to water molecules. A single phospholipid molecule has a phosphate group on one end called the head and two side-by-side chains of fatty acids that make up the lipid tails.
A The polar heads avoid water. When it is placed in water the phospholipid molecules form a. The head and the tail.
This means that they are part hydrophilic and part hydrophobic. Describe a phospholipid molecule and its interaction with water. Cell Membrane The cell membrane also called the plasma membrane consists of a lipid bilayer formed by phospholipids.
The phosphate group is negatively charged making the head polar and hydrophilic or water loving. The head is hydrophilic and the tail is hydrophobic. When placed in water hydrophobic molecules tend to form a ball or cluster.
Since these biological surfaces contain phospholipids the interaction of water with these lipids is of particular relevance to the biological function of the membrane surfaces. Describe a phospholipid -molecule and its interaction with water consists of a polar end containing a phosphate group that is hydro-phillic and a non-polar end containing two fatty acids that is hydrophobic. A- a phospholipid is less soluble in water because even though it has one end that is hydrophilic the end that is hydrophobic is larger.
Terms in this set 70 describe the structure of a phospholipid molecule. When placed in water the heads form hydrogen bonds with water molecules to form a complete sphere that shields the. B Phospholipids do not interact with water because water is polar and lipids are nonpolar.
Describe the phospholipid relationship to water.

Phospholipids Biology For Majors I

Solved D Short Answer Questions Meshning Gloss 1 What Is Chegg Com

Solved Uatehing Chromatids Are Held Together By A D Chegg Com
Comments
Post a Comment